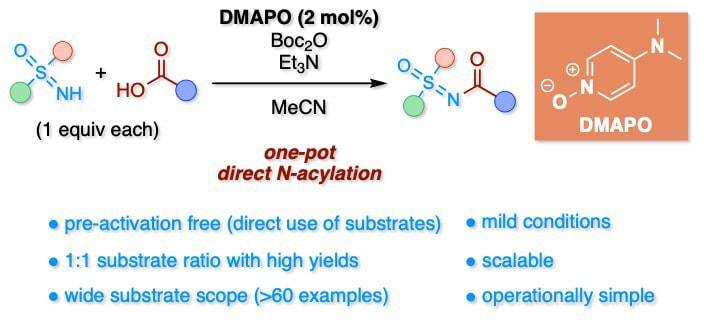
DMAPO/Boc2O-Mediated One-Pot Direct N-Acylation of Sulfoximines with Carboxylic Acids
Atsushi Umehara,* Sukenao Kawai, and Makoto Sasaki*
Europran Journal of Organic Chemistry 2025, e202500488
DOI: 10.1002/cctc.202201596
This report describes a general and simple method for the one-pot direct N-acylation of sulfoximines with carboxylic acids using the previously developed 4-(N,N-dimethylamino)pyridine N-oxide (DMAPO)/di-tert-butyl dicarbonate (Boc2O) system. The method can be performed under mild low-temperature reaction conditions and has a wide substrate scope. The present method is operationally simple, scalable, and practical; thus, it can find wide applications in both academic and industrial laboratories.

Simple and efficient one-pot amide formation reaction of less reactive nitrogen nucleophiles with carboxylic acids
Atsushi Umehara*
MEDCHEM NEWS, 2025, 35, 40–44.
In contrast to the significant progress of amide bond formations, the methods for N-acylation of low nucleophilic nitrogen heterocycles are limited due to their poor nucleophilicity. Herein we have developed one-pot direct N-acylation of low nucleophilic nitrogen heterocycles using DMAPO/Boc2O system. The new one-pot method, which does not involve pre- activation of substrates, enables the direct N-acylation of a wide variety of nitrogen nucleophiles with carboxylic acids. The new method also exhibits excellent functional group tolerance and broad substrate scope. As the present method is practical, operationally simple, and scalable, it should find wide applications in both academic and industrial laboratories.
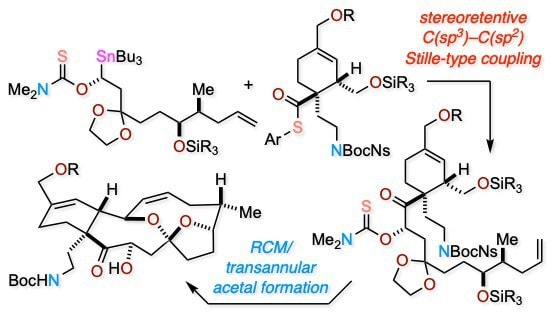
Stereocontrolled Synthesis of the Portimine Skeleton via Organocatalyst-Mediated Asymmetric Stannylation and Stereoretentive C(sp3)–C(sp2) Stille Coupling
Daisuke Sato, Makoto Sasaki,* Atsushi Umehara*
Organic Letters, 2025, 27, 942–947.
DOI: 10.1021/acs.orglett.4c04245
報道 from 日本経済新聞
Selected as Most Read Articles (02/2025)
Our efforts toward the synthesis of the marine natural product portimine are described. The key to the synthesis of the skeleton is a stereoretentive copper-catalyzed C(sp3)–C(sp2) Stille-type cross-coupling that enables the convergent assembly of functionalized fragments. The core skeleton of portimine was constructed via ring-closing metathesis and transannular acetal formation.
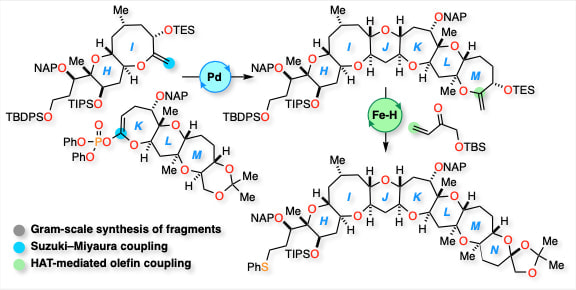
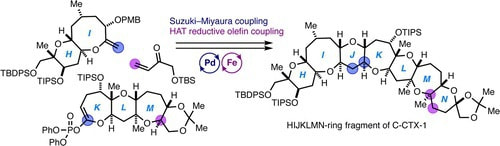
Convergent and Scalable Second-Generation Synthesis of Fully Functionalized HIJKLMN-Ring Segment of Caribbean Ciguatoxin C-CTX-1
Makoto Sasaki,* Miyu Ohba, Ako Murakami, Atsushi Umehara
The Journal of Organic Chemistry, 2024, 89, 18631–18639.
DOI: 10.1021/acs.joc.4c02723
報道 from 日本経済新聞 報道 from 産経ニュース 報道 from 産経新聞西日本版夕刊紙面
A highly convergent and scalable second-generation synthesis of the fully functionalized HIJKLMN-ring segment of Caribbean ciguatoxin C-CTX-1, the primary toxin responsible for ciguatera fish poisoning in the Caribbean Sea and the Northeast Atlantic regions, has been accomplished. Key aspects of the synthetic approach include the efficient syntheses of the HI- and KLM-ring fragments on gram scales, a convergent fragment coupling toward the HIJKLM-ring skeleton based on the Suzuki–Miyaura coupling strategy, and optimized iron hydride-catalyzed hydrogen atom transfer-mediated olefin coupling conditions for constructing the N-ring.
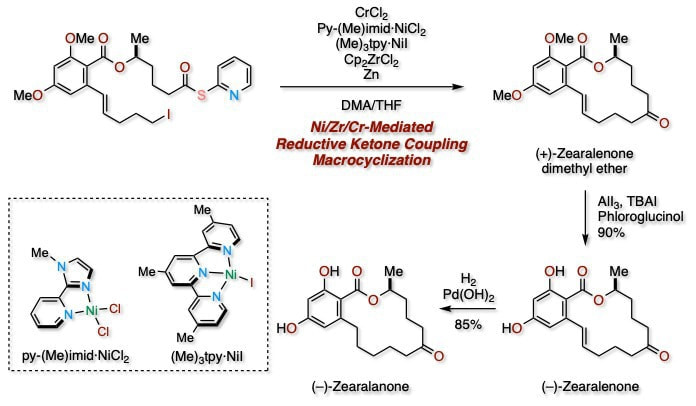
Total Synthesis of (–)-Zearalenone and (–)-Zearalanone: A Macrocyclization Strategy by Ni/Zr/Cr-Mediated Reductive Ketone Coupling
Atsushi Umehara,* Ko-hei Kawakita, Makoto Sasaki*
The Journal of Organic Chemistry, 2024, 89, 13800-13805.
DOI: 10.1021/acs.joc.4c01793
Selected as Most Read Articles (10/2024)
The total synthesis of the resorcylic acid lactones (−)-zearalenone and (−)-zearalanone is described. Our synthetic strategy relies on Ni-, Zr-, and Cr-mediated intramolecular reductive ketone coupling to create 14-membered macrolactones. Notably, the use of CrCl 2 in addition to Ni/Zr-mediated reductive ketone coupling conditions was key for the success of the macrocyclization.
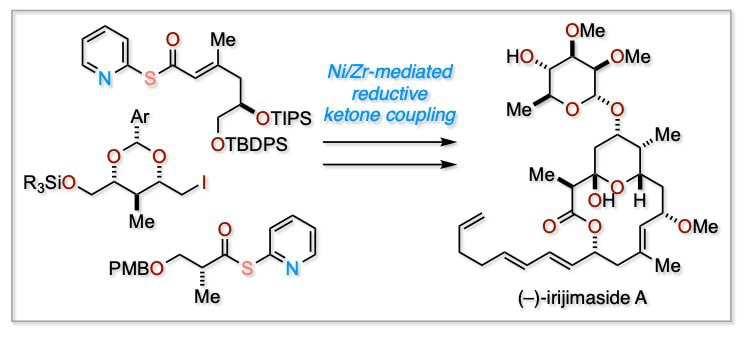
Total Synthesis of (–)-Irijimaside A Enabled by Ni/Zr-Mediated Reductive Ketone Coupling
Tomoya Suwa, Makoto Sasaki*, Atsushi Umehara*
Organic Letters, 2024, 26, 4377-4382.
DOI: 10.1021/acs.orglett.4c01367
報道 from 日本経済新聞
Selected as Most Read Articles (06/2024)
The total synthesis of marine macrolide glycoside (−)-irijimaside A is described. Key to the synthesis is the convergent fragment assembly enabled by nickel/zirconocene-mediated one-pot reductive ketone coupling. At the last stage of the synthesis, Stille coupling and glycosylation led to the first total synthesis of (−)-irijimaside A.
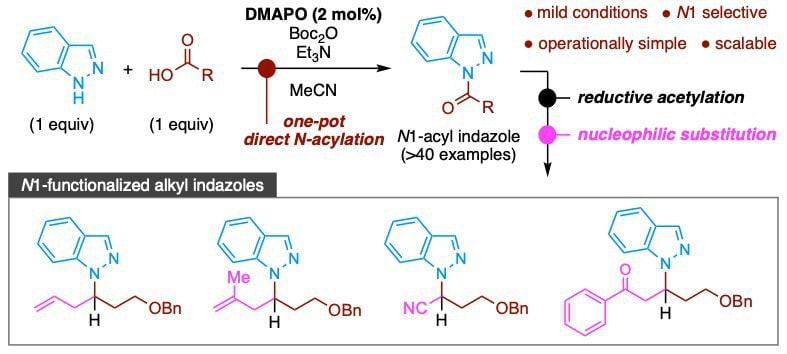
DMAPO/Boc2O-Mediated One-Pot Direct N1-Acylation of Indazole with Carboxylic Acids: A Practical Synthesis of N1-Functionalized Alkyl Indazoles
Atsushi Umehara,* Shimizu Soma, Makoto Sasaki*
Europran Journal of Organic Chemistry 2024, e202400123.
DOI: 10.1002/ejoc.202400123
This report describes the one-pot direct N-acylation of indazole with carboxylic acids using our previously developed 4-( N, N-dimethylamino)pyridine N-oxide (DMAPO)/di- tert-butyl dicarbonate (Boc 2O) system. This simple system provides N1-acyl indazoles in high yield with high N1 selectivities and does not require the use of activated derivatives of carboxylic acids or high temperatures. This new method exhibits a wide substrate scope (>40 examples). In addition, a new synthesis of N1-functionalized alkyl indazoles utilizing N1-acyl indazoles as starting materials was achieved. This stepwise protocol is useful for the selective synthesis of structurally diverse N1-functionalized alkyl indazoles, which are difficult to synthesize by other methods such as the Mitsunobu reaction and classical S N2 alkylation of indazole.
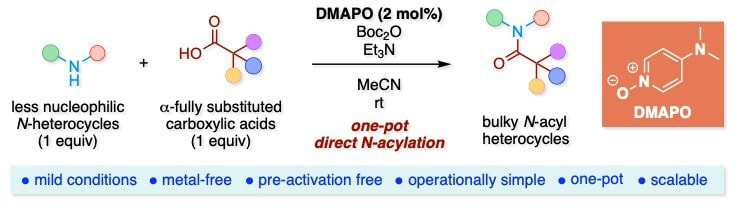
Synthesis of Bulky N-Acyl Heterocycles by DMAPO/Boc2O-Mediated One-Pot Direct N-Acylation of Less Nucleophilic N-Heterocycles with α-Fully Substituted Carboxylic Acids
Atsushi Umehara,* Shimizu Soma, Makoto Sasaki*
Advanced Synthesis & Catalysis 2023, 365, 2367-2376.
Top Viewed Article in Advanced Synthesis & Catalysis
Most Accessed Paper (07/2023), (08/2023) and (01/2025)
This report describes a general method for the one-pot direct N-acylation of N-heterocycles using our previously developed 4-(N,N-dimethylamino)pyridine N-oxide (DMAPO)/di-tert-butyl dicarbonate (Boc2O) system. This system enables one-pot N-acylation reactions to provide bulky N-acyl heterocycles starting from a wide variety of less nucleophilic N-heterocycles and sterically hindered α-fully substituted carboxylic acids in high yield. Moreover, this one-pot method does not involve pre-activation of substrates. The protocol has been successfully extended to one-pot N-acylation of 7-azaindoles.

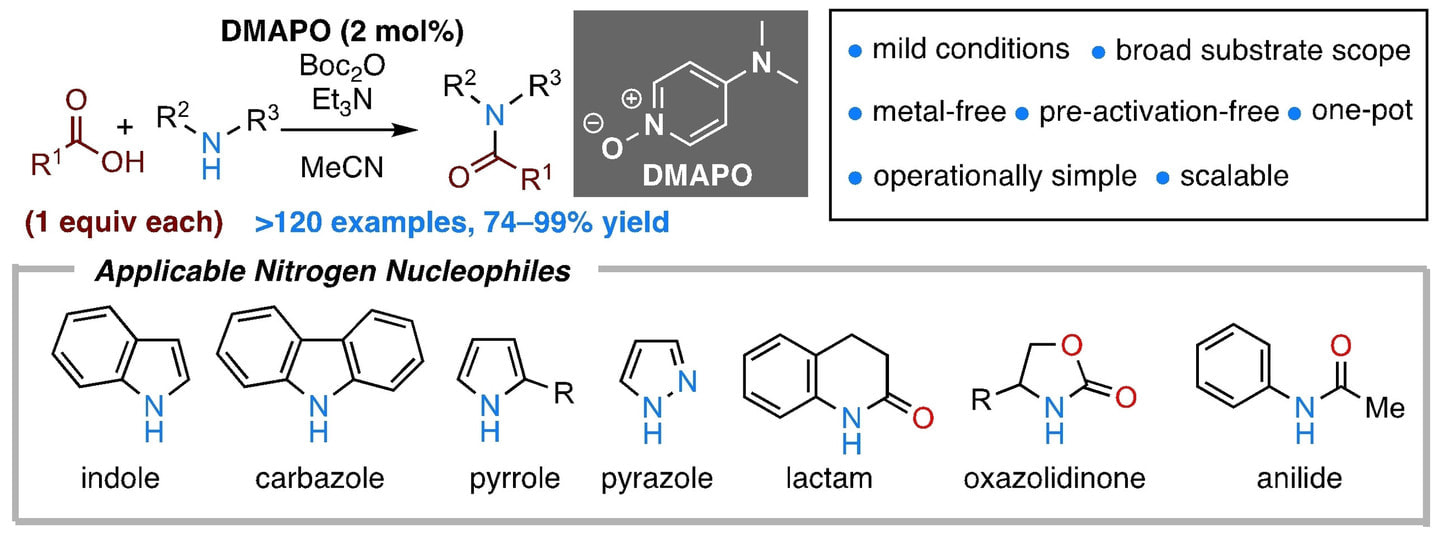
DMAPO/Boc2O-Mediated One-Pot Direct N-Acylation of Less Nucleophilic N-Heterocycles with Carboxylic Acids
Atsushi Umehara,* Soma Shimizu, Makoto Sasaki*
ChemCatChem 2023, 15, e202201596.
Most Accessed Paper (02/2023), (04/2023), (05/2023), (06/2023), (07/2023), (08/2023), (09/2023), (10/2023), (11/2023), (12/2023), (01/2024), (02/2024), (04/2024), (05/2024), (06/2024), (07/2024), (08/2024), (01/2025), (02/2025) and (03/2025)
In contrast to the considerable progress in the development of methodologies for amide bond formation in amines, the development of direct N-acylation of less nucleophilic N-heterocycles and amides with carboxylic acids is still challenging. In this report, we describe the direct N-acylation of less nucleophilic heterocycles and amides with carboxylic acids promoted by the 4-( N, N-dimethylamino)pyridine N-oxide (DMAPO)/di- tert-butyl dicarbonate (Boc 2O) system. The new one-pot method, which does not involve pre-activation of substrates, enables the direct N-acylation of a wide variety of nitrogen nucleophiles such as indole, carbazole, pyrrole, pyrazole, lactam, oxazolidinone, and anilide with carboxylic acids in high yield. The new method also exhibits excellent functional group tolerance and broad substrate scope. As the present method is practical, operationally simple, and scalable, it should find wide applications in both academic and industrial laboratories.

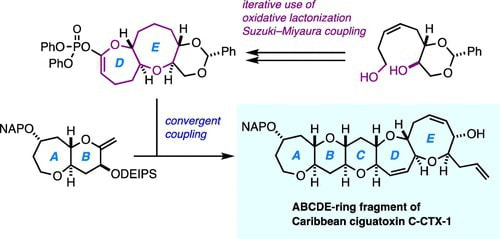
Convergent and Scalable Synthesis of the ABCDE-Ring Fragment of Caribbean Ciguatoxin C-CTX-1
Makoto Sasaki*, Miku Seida, Atsushi Umehara
J. Org. Chem. 2023, 88, 403-418
研究科のHPで取りあげてもらいました。
Convergent and scalable synthesis of the ABCDE-ring fragment of Caribbean ciguatoxin C-CTX-1, the major causative toxin for ciguatera poisoning in the Caribbean Sea and the Northeast Atlantic areas, is described in detail. The key features of the synthesis include an iterative use of 2,2,6,6-tetramethyl piperidine 1-oxyl (TEMPO)/PhI(OAc) 2-mediated oxidative lactonization and Suzuki–Miyaura coupling en route to the DE-ring system and a convergent fragment coupling to form the ABCDE-ring skeleton via the Suzuki–Miyaura coupling strategy.
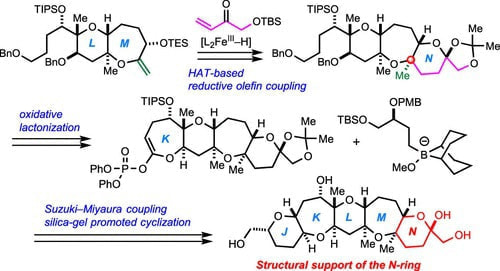
Convergent Synthesis of the HIJKLMN-Ring Fragment of Caribbean Ciguatoxin C-CTX-1 by a Late-Stage Reductive Olefin Coupling Approach
Makoto Sasaki*, Kotaro Iwasaki, Keisuke Arai, Naoya Hamada, Atsushi Umehara
Bull. Chem. Soc. Jpn. 2022, 95, 819–824.
プレスリリース from 東北大院生命科学研究科
プレスリリース from 東北大本部
The convergent synthesis of the HIJKLMN-ring fragment of Caribbean ciguatoxin C-CTX-1, the major causative toxin for ciguatera fish poisoning in the Caribbean Sea and the Northeast Atlantic areas, is disclosed. The synthesis features a late-stage iron-catalyzed hydrogen atom transfer-initiated reductive olefin coupling to install the N-ring and a Suzuki–Miyaura coupling/thioacetalization strategy for the convergent assembly of the hexacyclic HIJKLM-ring skeleton.The convergent synthesis of the HIJKLMN-ring fragment of Caribbean ciguatoxin C-CTX-1, the major causative toxin for ciguatera fish poisoning in the Caribbean Sea and the Northeast Atlantic areas, is disclosed. Highlights of the synthesis are Suzuki–Miyaura coupling/thioacetalization strategy for convergent assembly of the HIJKLM-ring skeleton and a late-stage hydrogen atom transfer-initiated reductive olefin coupling to construct the terminal N-ring.
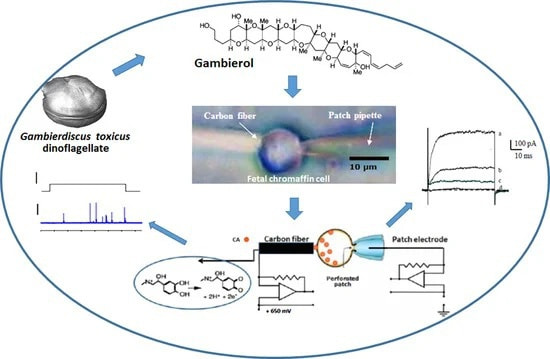
Gambierol Blocks a K+ Current Fraction without Affecting
Catecholamine Release in Rat Fetal Adrenomedullary Cultured Chromaffin Cells
Evelyne Benoit, Sébastien Schlumberger, Jordi Molgó, Makoto Sasaki, Haruhiko Fuwa, Roland Bournaud
Toxins 2022, 14, 254.
Determination of the Toxicity Equivalency Factors for Ciguatoxins using Human Sodium Channels
Sandra Raposo-Garcia, M. Carmen Louzao, Haruhiko Fuwa, Makoto Sasaki, Carmen Vale, Luis M. Botana
Food Chem. Toxicol. 2022, 160, 112812
Synthesis and Structural Implication of the JKLMN-Ring Fragment of Caribbean Ciguatoxin C-CTX-1
Makoto Sasaki*, Kotaro Iwasaki, and Keisuke Arai
J. Org. Chem. 2021. 86, 4580
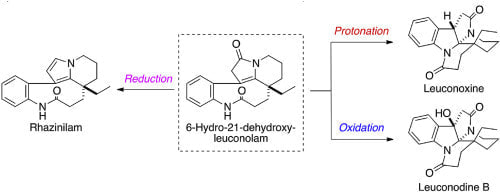
Synthesis of leuconoxine, leuconodine B, and rhazinilam by transformation of melodinine E via 6-hydro-21-dehydroxyleuconolam
Astushi Umehara, Hirofumi Ueda, Hidetoshi Tokuyama
Tetrahedron, 2021, 79, 131809
Unified Total Synthesis of (−)-Enigmazole A and (−)-15-O-Methylenigmazole A
Keisuke Sakurai, Keita Sakamoto, Makoto Sasaki and Haruhiko Fuwa
Chem. Asian J. 2020, 15, 3494.
Gambierol Potently Increases Evoked Quantal Transmitter Release and Reverses Pre- and Post-Synaptic Blockade at Vertebrate NeuromuscularJunctions
Jordi Molgó, Sébastien Schlumberger, Makoto Sasaki, Haruhiko Fuwa, M. Carmen Louzao, Luis M. Botana, Denis Servent, and Evelyne Benoit
Neuroscience 2020, 439, 106–116.
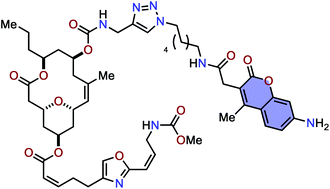
Fluorescence-labeled neopeltolide derivatives for subcellular localization imaging
Shota Yanagi, Tomoya Sugai, Takuma Noguchi, Masato Kawakami, Makoto Sasaki, Shinsuke Niwa, Asako Sugimoto and Haruhiko Fuwa
Org. Biomol. Chem. 2019, 17, 6771
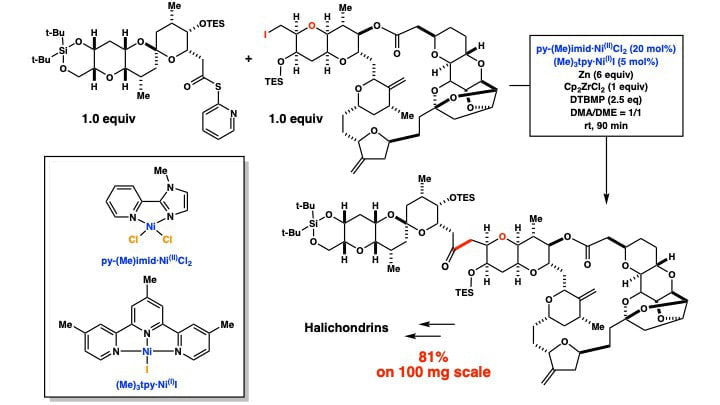
Further Studies on Ni/Zr-mediated One-pot Ketone Synthesis: Use of a Mixture of NiI- and NiII-catalysts Greatly Improves the Molar Ratio of Coupling Partners
Atsushi Umehara and Yoshito Kishi
Chem. Lett. 2019, 48, 947–950
A new Ni/Zr-mediated one-pot ketone synthesis is developed, with use of a mixture of (Me) 3tpy·Ni II- and py-(Me)imid·Ni IICl 2-catalysts. The Ni I-catalyst selectively activates iodides, whereas the Ni II-catalyst activates thio-pyridine esters. An adjustment of a relative loading of the two catalysts allows to tune the relative rate of the two activations and trap a short-lived radical intermediate(s) efficiently. Thus, the new method makes one-pot ketone synthesis highly effective even with a 1:1 mixture of the coupling partners. The synthetic value of the new method is demonstrated with the C-C bond formation at the final stage of a convergent halichondrin-synthesis.
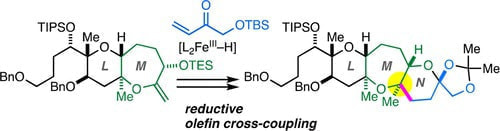
Studies toward the Total Synthesis of Caribbean Ciguatoxin C‐CTX-1: Synthesis of the LMN-Ring Fragment through Reductive Olefin Cross-Coupling
Makoto Sasaki, Kotaro Iwasaki, and Keisuke Arai
Org. Lett. 2018, 20, 7163−7166
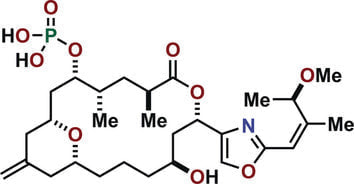
Total Synthesis of (−)‐Enigmazole A
Keisuke Sakurai, Makoto Sasaki, and Haruhiko Fuwa
Angew. Chem. Int. Ed. 2018, 57, 5143 –5146
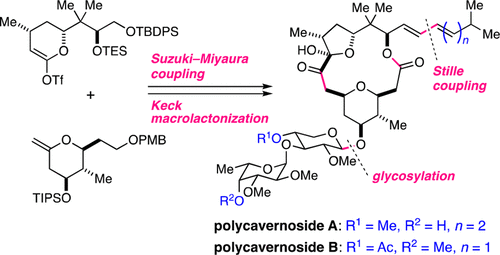
Total Synthesis of Polycavernosides A and B, Two Lethal Toxins from Red Alga
Kotaro Iwasaki, Satori Sasaki, Yusuke Kasai, Yuki Kawashima, Shohei Sasaki, Takanori Ito, Mari Yotsu-Yamashita, and Makoto Sasaki
J. Org. Chem. 2017, 82, 13204−13219
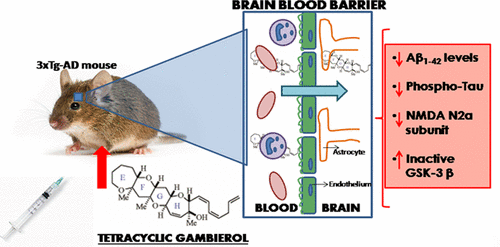
Tetracyclic Truncated Analogue of the Marine Toxin Gambierol Modifies NMDA, Tau, and Amyloid β Expression in Mice Brains: Implications in AD Pathology
Eva Alonso, Andreś C. Vieira, Ineś Rodriguez, Rebeca Alvariño, Sandra Gegunde, Haruhiko Fuwa, Yuto Suga, Makoto Sasaki, Amparo Alfonso, José Manuel Cifuentes, and Luis M. Botana
ACS Chem. Neurosci. 2017, 8, 1358−1367




















































